Overview
MATRIX: A USAID Project to Advance the Research and Development of Innovative HIV Prevention Products for Women
MATRIX is a nine-year program funded by the U.S. Agency for International Development (USAID) in 2021 with a mission to expedite the research and development of HIV prevention products for women that in addition to being safe and effective, will be acceptable, affordable, scalable and deliverable in the settings where they are needed most.
MATRIX activities are focused primarily on the early research and development of products, which involves both pre-clinical research – the laboratory and animal studies needed to support a product’s evaluation in humans – and the first clinical trials of products, as well as evaluating the safety of approved products in pregnant women. Through its North-South Partnerships, MATRIX also aims to strengthen the research and development capacity of African investigators in order to facilitate full and sustainable ownership of this work.
MATRIX is being implemented by Magee-Womens Research Institute (MWRI) in collaboration with nearly 20 partner organizations based in Kenya, Lesotho, South Africa, Uganda, the United States and Zimbabwe. Leading the project is Sharon Hillier, Ph.D., of MWRI and the University of Pittsburgh, USA, with Thesla Palanee-Phillips, Ph.D., from Wits RHI and University of Witwatersrand, South Africa, serving as deputy director. Collectively, MATRIX partners have expertise across multiple fields, including drug formulation, drug delivery and product development; clinical trials design and implementation; human-centered design and socio-behavioral research; market strategy and business case development; capacity strengthening; and stakeholder engagement.
How is MATRIX unique?
Early research and development is an unpredictable process – of hundreds of potential products being evaluated in pre-clinical research, only a handful will make it into early-phase clinical studies, and fewer still can expect to progress all the way to regulatory approval. To increase the odds of success, MATRIX is taking a unique approach that prioritizes only those products that have the greatest chance to succeed and add value. It’s not enough that laboratory and animal studies suggest a product will be safe and effective in humans. There must also be evidence that potential end-users will likely use the product; it can be manufactured and distributed locally and at low cost; it will be easy to deliver, with minimal burden on healthcare systems; will meet the needs of Ministries of Health and national prevention programs; and serve to enhance the existing toolbox of options. It’s a deliberately nimble and responsive approach to product development guided by clearly defined milestones and “Go/No-Go” criteria,” with an independent scientific advisory group that provides unbiased assessment of a product’s progress in meeting its timelines and milestones as well as considers developments within the broader field.
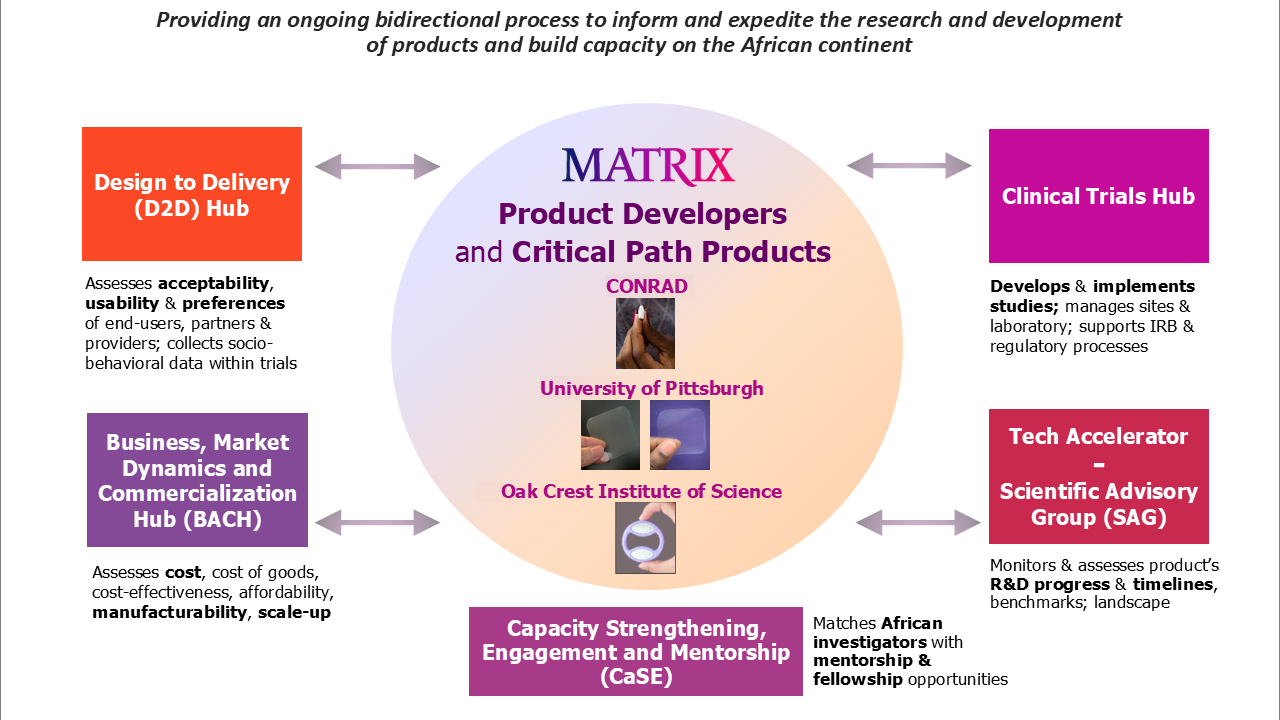
The MATRIX structure consists of five activity hubs that support product developers via a bidirectional process to inform and expedite the research and drug development of products and build capacity on the African continent. For instance, the Design to Delivery (D2D) Hub provides insight into the needs and preferences of end-users, male partners and healthcare workers; the Business, Market Dynamics and Commercialization Hub (BACH) looks at cost, manufacturability, deliverability and scale-up, and the Clinical Trials Hub designs and implements observational studies and Phase 1 trials in Africa – in parallel with the United States – to answer questions about a product’s safety, acceptability and usability, particularly among the intended populations of women the products are being designed for.
The MATRIX Product Pipeline
In order to meet the diverse needs of women, MATRIX aims to develop a range of HIV prevention products. The current portfolio of critical path products includes a short-acting, on-demand product intended to be used at the time of sex (TAF/EVG fast-dissolving insert); and three products for monthly protection: dapivirine vaginal film, a dual-purpose vaginal film; and a dual-purpose vaginal ring containing novel agents. MATRIX is also supporting research seeking to develop products that could potentially provide protection for up to a year.
MATRIX Clinical Trials and Studies
In December 2024, MATRIX completed its first Phase 1 trial, which evaluated the safety and drug pharmacokinetics and pharmacodynamics (how and where drug is taken up in the body) of the TAF/EVG fast-dissolving vaginal insert, with results expected in 2025 and a second Phase 1 study expected to follow soon after. MATRIX has also completed a study that evaluated the acceptability and usability of two placebo prototype monthly vaginal films. Results, due mid-2025, will determine the film to be used in a first-in-human trial of the monthly dapivirine film that’s expected to begin late 2025. A similar study of two prototype monthly vaginal rings is expected to be completed early 2025, the results of which will inform next steps for the ring’s development.
In addition, MATRIX is conducting a study that will contribute important safety data on the use of cabotegravir long-acting injection (CAB-LA), the monthly dapivirine vaginal ring and daily oral pre-exposure prophylaxis (PrEP) during pregnancy. The MATRIX-007 (CARE PrEP) study is being conducted in collaboration with Maximizing Options to Advance Informed Choice for HIV Prevention (MOSAIC), a five-year program funded by the U.S. President’s Fund for AIDS Relief through USAID.
