Cabotegravir Pellet Implant
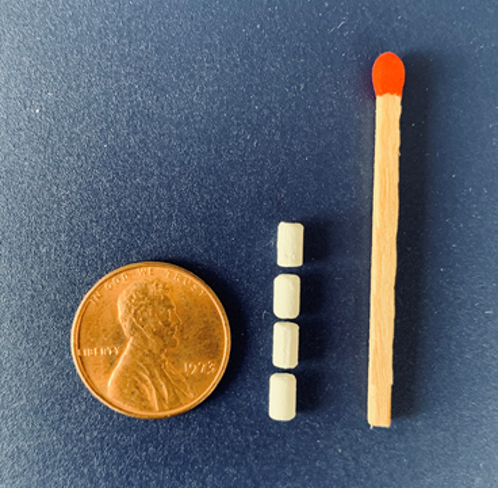
The cabotegravir pellet implant developed by CONRAD is a high priority, low-cost pellet-based subdermal implant system with customized inserter for rapid, safe, and low-cost use by minimally trained health care providers that may provide prophylactic levels of cabotegravir for up to 9-12 months. This implant can be removed, if needed, with the active pharmaceutical ingredient having a potentially shorter pharmacokinetics tail. Within this collaborative, the lead formulation will be optimized and tested for safety, pharmacokinetics and efficacy in animal studies. Investigational New Drug enabling studies will be guided by a pre-investigational new drug meeting which will also provide an opportunity to confirm regulatory strategy (proposed bioequivalence path) and clinical testing plans in key populations in Sub-Saharan Africa.
